How To Get Hydrogen From Water Without Electricity
A team of scientists at the RIKEN Center for Sustainable Resources Scientific discipline (CSRS) in Japan headed by Ryuhei Nakamura has discovered a new practical and sustainable arroyo for generating hydrogen from h2o. On the contrary to electric current approaches, the new method does non need rare metals that are short in supply or costly.
Instead, hydrogen for agricultural fertilizers and fuel cells can be now produced using manganese and cobalt, two equally mutual metals. The inquiry was published in Nature Catalysis.
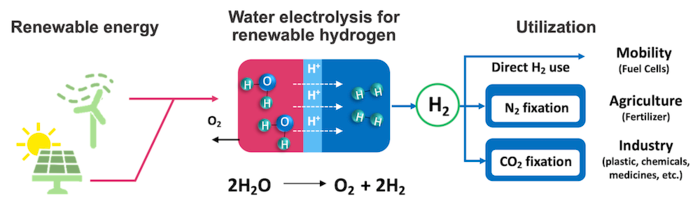
Hydrogen is a clean fuel that produces water as its only by-product, unlike traditional fossil fuels that produce carbon dioxide on combustion. The free energy filigree tin can be made renewable, clean and sustainable if hydrogen can be retrieved from water with the apply of renewable electricity.
In addition, hydrogen is the vital ingredient required in the production of ammonia that is employed in nearly all synthetic fertilizers. However, instead of extracting hydrogen cleanly from h2o, ammonia plants are now utilizing fossil fuels to generate the hydrogen they require.
The unsustainable and expensive nature of electrolysis — the hydrogen extraction procedure — could be ane reason.
This is primarily due to a lack of good catalysts. In addition to beingness able to withstand the harsh acidic environment, the catalyst must be very agile. If not, the amount of electricity needed for the reaction to produce a given amount of hydrogen soars, and with it, so does the toll.
Ryuhei Nakamura, Study Pb, Heart for Sustainable Resource Scientific discipline, RIKEN
Now, rare metals like platinum and iridium are the most agile catalysts for h2o electrolysis, creating a dilemma, equally they are costly and regarded as "endangered species" among metals.
Correct now, iridium production of near 800 years' worth — an amount which might be incommunicable to exist — would exist required for switching the earth to hydrogen fuel. On the contrary, abundant metals, like iron and nickel, are not active enough and dissolve immediately in the surroundings of harsh acidic electrolysis.
The scientists considered mixed cobalt and manganese oxides in their quest for a better goad. Cobalt oxides could be agile for the required reaction but corrode very speedily in the acidic environment. Manganese oxides are more stable in comparison just are not that active.
By mixing them, the scientists expected to benefit from their complementary properties. The scientists also considered the high electric current density requirement for practical application outside the lab environment.
For industrial calibration hydrogen product, we needed to set our study's target current density to about 10 to 100 times college than what has been used in past experiments. The high currents led to a number of problems such as physical decomposition of the catalyst.
Shuang Kong, Co-Offset Author, Center for Sustainable Resource Science, RIKEN
Consequently, they overcame these problems, through trial and error, and establish a stable and active catalyst by introducing manganese into the spinel lattice of Co3Ofour, creating the mixed cobalt manganese oxide Co2MnOfour.
Upon assessment, information technology was revealed that CoiiMnO4 functions very well. Activation levels were nigh to those of advanced iridium oxides. In addition, the new goad remained for more than two months at a electric current density of 200 milliamperes per square centimeter, which could make it efficient for practical use.
The new electrocatalyst could be a game-changer compared with other non-rare metal catalysts that tin merely normally terminal a few days or weeks at much lower electric current densities.
Nosotros accept achieved what has eluded scientists for decades. Hydrogen product using a highly agile and stable catalyst made from abundant metals. In the long run, we believe that this is a huge step towards creating a sustainable hydrogen economy. Like other renewable technologies such every bit solar cells and wind power, we wait the toll of green hydrogen technology to collapse in the near future equally more advances are made.
Ailong Li, Study Co-Starting time Author, Centre for Sustainable Resource Science, RIKEN
Finding means to expand the lifetime of the new catalyst and enhance its activity levels further is the adjacent pace in lab. "In that location is always room for improvement," concludes Nakamura, "and we go along to strive for a not-rare metal goad that matches the performance of electric current iridium and platinum catalysts."
Journal Reference:
Li, A., et al. (2022) Enhancing the stability of cobalt spinel oxide towards sustainable oxygen evolution in acrid. Nature Catalysis. doi.org/ten.1038/s41929-021-00732-9.
Source: https://www.riken.jp/en/
How To Get Hydrogen From Water Without Electricity,
Source: https://www.azom.com/news.aspx?newsID=58250
Posted by: scottaskins90.blogspot.com


0 Response to "How To Get Hydrogen From Water Without Electricity"
Post a Comment